COTTON-LIKE SYNTHETIC BONE FILLER ReBOSSIS
What is ReBOSSIS?
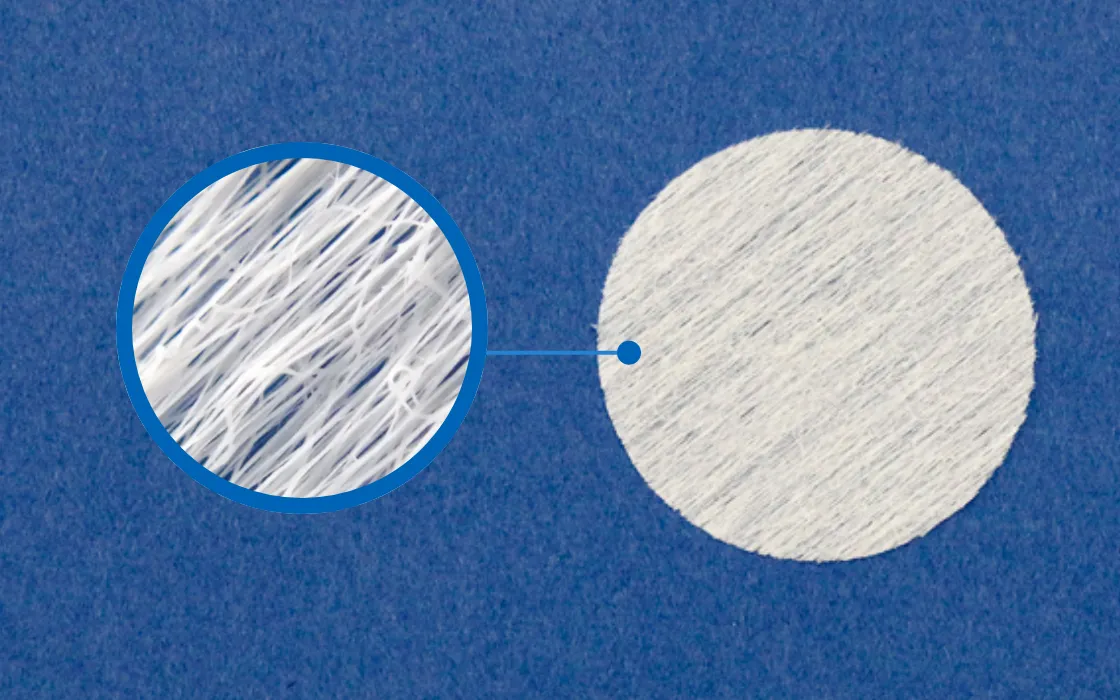
ReBOSSIS is a cotton-like synthetic bone void filler composed primarily of β-TCP and bioabsorbable polymers. Unlike conventional block-shaped synthetic bones that require customization to fit defect sizes, or granular synthetic bones that may spill from the filled area, ReBOSSIS’s cotton form offers exceptional ease of handling.
In addition to its manageability, ReBOSSIS provides elasticity and resilience, setting it apart from existing synthetic bone materials. This allows for quick and reliable implantation of defects, regardless of their location or size. Moreover, ReBOSSIS fits securely, stays in place, and is completely replaced by the patient’s own bone after treatment.
High Operability
Easily molds to fit the size and shape of defect areas
Simple to mix with autologous bone
Excellent Filling Properties
Effectively fills defect areas
Minimizes spillage from the filling site
Stable Absorption
Maintains blood and bone marrow fluid retention for optimal absorption

Fine Fiber Structure
Electrospinning technology creates a refined fiber structure
Acts as a scaffold for osteoblasts, facilitating bone regeneration.
High Porosity
Allows easy infiltration of cells and growth factors essential for bone formation
Absorption & Replacement
Gradually absorbed and replaced by new bone during osteogenesis
The Journey and History of ReBOSSIS
The foundational technology for REBOSSIS was developed by a research group led by Professor Toshihiro Kasuga, now Professor Emeritus at Nagoya Institute of Technology. The research began in 2011, initially aimed at creating synthetic bone for dental implants. The main components were the same as REBOSSIS: β-TCP and polymers, but in nonwoven fabric form.
Through the process of electrospinning, the material was eventually transformed into fibers. However, it took considerable trial and error to create the extremely fine cotton-like fibers characteristic of REBOSSIS. Achieving the optimal shape to activate osteoblasts, including determining the right fiber thickness, required significant time and effort.
The current cotton-like synthetic bone void filler, REBOSSIS, is the result of further improvements made by ORTHOREBIRTH, building upon the technology developed by the Nagoya Institute of Technology research team.
In August 2014, we sought approval from the U.S. Food and Drug Administration (FDA), and by October 2014, we achieved FDA 510(k) Clearance in the field of Trauma. With these approvals, ReBOSSIS was introduced to the U.S. market in April 2015.
In December 2017, we obtained clearance for use in the spinal area, allowing ReBOSSIS to be utilized in a broader range of orthopedic surgeries.
For the Japanese market, ReBOSSIS received manufacturing and sales approval in March 2021, and the product was launched under the name ReBOSSIS-J in September of the same year.

ReBOSSIS Product Line
ReBOSSIS is sold under the name “ReBOSSIS” in overseas markets such as the United States and Taiwan. However, starting from Spring 2024, the American company KYOCERA Medical Technologies, Inc. has renamed the product to BioCera Fibers. For the Japanese market, orthopedic products are labeled as ReBOSSIS-J, while products for neurosurgery, oral surgery, and plastic surgery are named according to their respective distributors as ReBOSSIS-MS, ReBOSSIS-MT, ReBOSSIS-MK, and ReBOSSIS-MC under the ReBOSSIS-M Series.
Collectively, these products are known as the ReBOSSIS Series. While all ReBOSSIS Series products share the same basic components, the products for the Japanese market were tailored to meet specific local requirements, offering enhancements based on regional needs.
ReBOSSIS Product Line
Country of Sales: Japan
Cell Culture Substrate
Stem Cell Extraction Culture Sheet for Research
Efficient Cell Culturing and Cost Reduction
This innovation is
the crystallization of
our bioabsorbable
microfiber technology
Our microfiber scaffolding material manufacturing technology has garnered significant attention from universities, research institutions, and companies involved in regenerative medicine.
The already commercialized “Stem Cell Extraction Culture Sheet for Research” has been shown to accelerate cell proliferation when used as a scaffolding material for cell culture. Its high bioabsorbability allows for the cultured cell sheets to be implanted directly into the body. This innovative approach is expected to revolutionize cell culture, which has traditionally been time-consuming and costly.







