Country of Sales: JAPAN
For Maxillofacial and Plastic Surgery:ReBOSSIS-MT

Product Information
- Brand Name:
-
ReBOSSIS-MS
ReBOSORB
- Approval Number(Medical Device):
-
30300BZX0095A03
- Approval Date:
-
August 5th, 2022
Reach Out to Our Distributor
- Sales Consignment:
- Teijin Medical Technologies Co., Ltd.
- Address:
-
13th Floor, Osaka Mitsui Bussan Bldg.,2-3-33,
Nakanoshima, Kita-ku, Osaka 530-0005, Japan
- TEL:
-
+81-86-279-6278
- FAX:
-
+81-86-279-9510
ReBOSSIS Product Line
Country of Sales: Japan
Cell Culture Substrate
Stem Cell Extraction Culture Sheet for Research
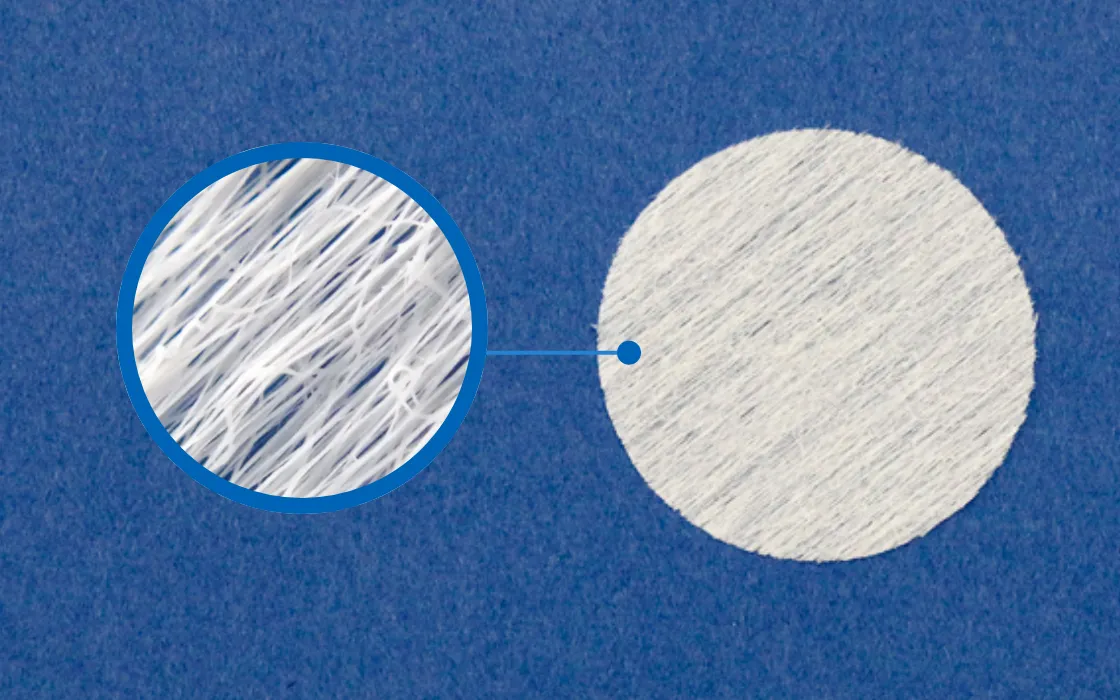
Efficient Cell Culturing and Cost Reduction
This innovation is
the crystallization of
our bioabsorbable
microfiber technology
Our microfiber scaffolding material manufacturing technology has garnered significant attention from universities, research institutions, and companies involved in regenerative medicine.
The already commercialized “Stem Cell Extraction Culture Sheet for Research” has been shown to accelerate cell proliferation when used as a scaffolding material for cell culture. Its high bioabsorbability allows for the cultured cell sheets to be implanted directly into the body. This innovative approach is expected to revolutionize cell culture, which has traditionally been time-consuming and costly.






